Adipose-derived mesenchymal stem cell (ADMSC) injections are a beneficial and growing therapy for musculoskeletal issues such as hip, knee and ankle osteoarthritis. As one of the leading clinics using ADMSCs, Oregon Regenerative Medicine has been perfecting this technique for over 15 years. How long can patients anticipate joint pain relief from this therapy to last? A recent study provides valuable insights.
THE STUDY
This fresh research provides encouraging data to support the positive results of ADMSC. An article published in the journal Stem Cells Translation Medicine1 summarizes this five-year retrospective study of patients who received ADMSC for their osteoarthritis of the knee (KOA).
The study used pain scales and MRI imaging to measure the treatment effectiveness as well as measure any adverse effects post treatment. This study is unique in that it provides extended follow-up of six months, one, two, three, four, and five years post procedure. Most ADMSC studies are limited to six twelve-month follow up assessments.2,3
STUDY RESULTS
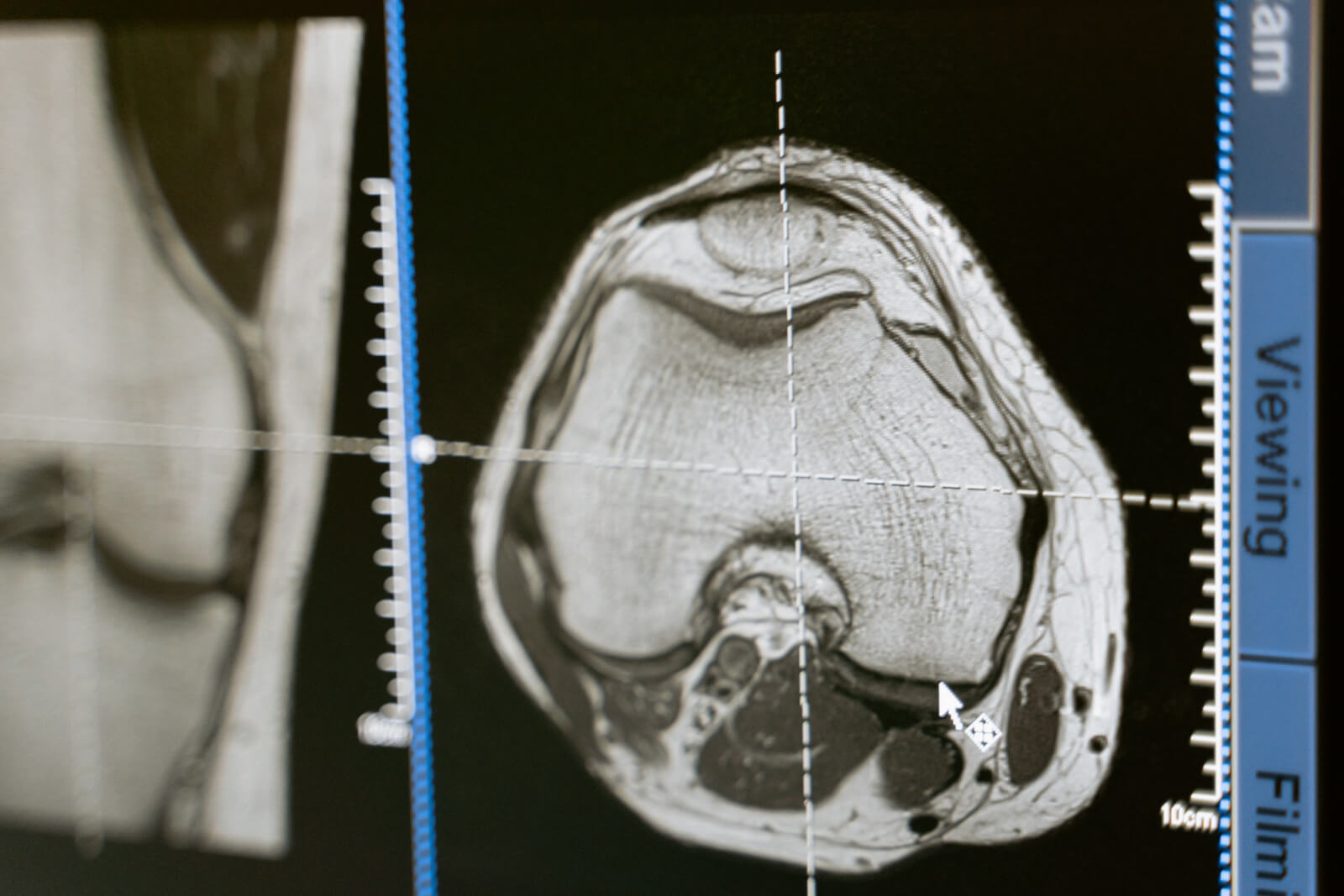
Each of the stem cell injection therapy study’s eleven KOA patients (8 females and 3 males) had significant pain reduction and sustained improvement from six months to three years post procedure. While some pain and swelling were noted, no severe adverse effects were noted. The inner aspects of the knee, the area most affected in KOA, revealed cartilage improvement post ADMSC injection. Cartilage defect areas were restored and the irregular surface of the articular cartilage changed into a congruent surface at 2, 3, 4, and 5 years after the injection of ADMSCs. Based on MRI analysis, years two and three were the most beneficial for improvements in cartilage reduction, bone marrow edema and synovitis.
Synovitis (inflammation of the synovial membranes) is a frequent culprit of perpetual joint inflammation and progressive joint degradation. ADMSC’s secrete cytokines, which are anti-inflammatory in nature, overriding the synovitis process and reducing the osteoarthritis thickening of the synovial membranes and preventing cartilage degradation in the joint4.
STUDY IMPERFECTIONS
This study was not perfect. Ideally, the authors would have administered another injection of Platelet Rich Plasma, as Oregon Regenerative Medicine does, as a “booster” to strengthen the healing process. Although high resolution 3 Tesla MRIs were obtained at 2, 3, 4, and 5 years, a one-year 3T MRI image was not obtained, losing valuable evaluation during a critical time of healing.
Unfortunately, while the study hosts allowed the participants to receive non-steroidal, anti-inflammatory drugs and hyaluronic acid injections during the 5-year period, they restricted PRP injections. At ORM, we administer follow-up PRP injections which expedite the healing process with growth factors, further enhancing the healing process and pain reduction.
CONCLUSION
Despite this study’s “imperfections”, it is encouraging to see a study further validating what we have seen clinically at Oregon Regenerative Medicine. None of the participants opted for a surgical intervention during this 5 year study. As an alternative to a knee replacement, this study reveals that ADMSC injections definitely have “legs” to stand on.
Dr. Stacey Guggino graduated from the National College of Natural Medicine in Portland, Oregon with a Doctorate in Naturopathy and a Master’s degree in Oriental Medicine. For the past 12 years, she has specialized in treating pain and sports injuries with acupuncture and prolotherapy. Stacey has also studied and practiced aesthetic medicine for 11 years.
- Kim KI, Lee WS, Kim JH, Bae JK, Jin W. Safety and Efficacy of the Intra-articular Injection of Mesenchymal Stem Cells for the Treatment of Osteoarthritic Knee: A 5-Year Follow-up Study. Stem Cells Transl Med. 2022 Jun 22;11(6):586-596. doi: 10.1093/stcltm/szac024. PMID: 35567774; PMCID: PMC9216498.
- Jo CH, Chai JW, Jeong EC, Oh S, Shin JS, Shim H, Yoon KS. Intra-articular Injection of Mesenchymal Stem Cells for the Treatment of Osteoarthritis of the Knee: A 2-Year Follow-up Study. Am J Sports Med. 2017 Oct;45(12):2774-2783. doi: 10.1177/0363546517716641. Epub 2017 Jul 26. PMID: 28746812.
- Lee WS, Kim HJ, Kim KI, Kim GB, Jin W. Intra-Articular Injection of Autologous Adipose Tissue-Derived Mesenchymal Stem Cells for the Treatment of Knee Osteoarthritis: A Phase IIb, Randomized, Placebo-Controlled Clinical Trial. Stem Cells Transl Med. 2019 Jun;8(6):504-511. doi: 10.1002/sctm.18-0122. Epub 2019 Mar 5. PMID: 30835956; PMCID: PMC6525553.
- van Lent PL, van den Berg WB. Mesenchymal stem cell therapy in osteoarthritis: advanced tissue repair or intervention with smouldering synovial activation? Arthritis Res Ther. 2013 Mar 20;15(2):112. doi: 10.1186/ar4190. PMID: 23521980; PMCID: PMC3672811.
Photo by Pawel Czerwinski on Unsplash